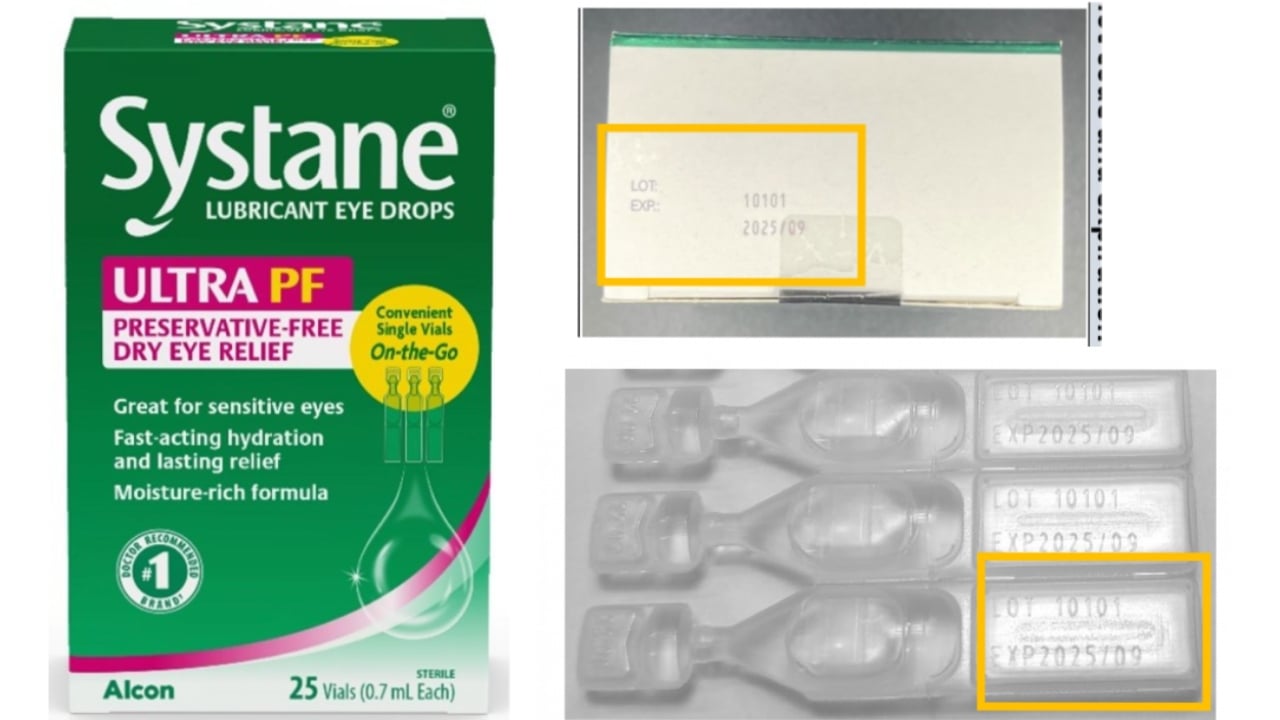
Alcon Laboratories has initiated a voluntary recall of one lot of Systane Lubricant Eye Drops Ultra PF after confirming fungal contamination in a sealed vial. The recall affects lot number 10101 with an expiration date of September 2025, distributed nationwide through major retailers including Publix, CVS, Walgreens, and Target.
The recall emerged after a consumer discovered foreign material in a sealed single-use vial, which laboratory analysis confirmed as fungal in nature. While no adverse reactions have been reported to date, the presence of fungal contamination raises serious concerns about quality control measures in ophthalmic product manufacturing.
“The presence of foreign material appears to be isolated to the single unit returned by a customer,” stated Steven Smith, Alcon spokesperson. The company initiated the recall “out of an abundance of caution to prioritize consumer safety.”
The affected product comes in a green and pink carton containing 25 sterile, single-use LDPE plastic vials (NDC 0065-1432-06, UPC 300651432060). These preservative-free eye drops are commonly used for temporary relief of burning and irritation associated with dry eye symptoms.
Health Risks and Technical Analysis
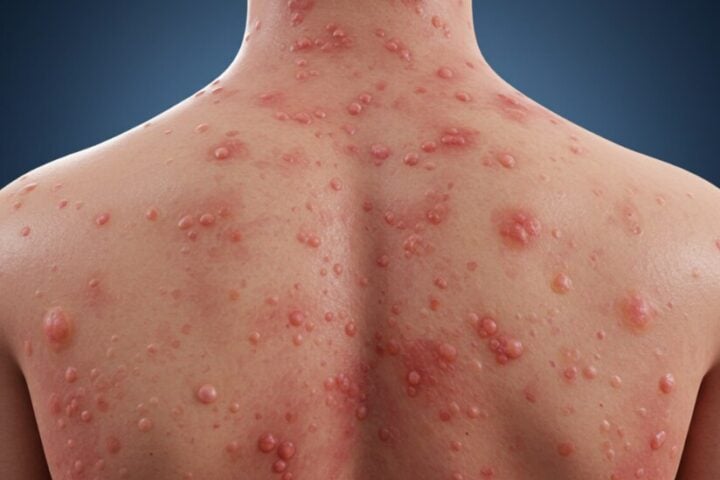
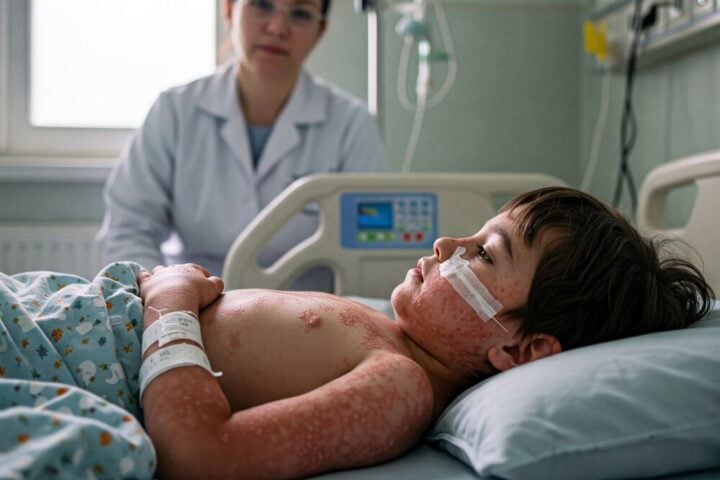
Ophthalmologists stress that fungal contamination in ophthalmic products presents severe risks. The time window for infection development ranges from several days to multiple weeks post-exposure. Medical experts note that early detection becomes crucial as these infections can progress rapidly if left untreated.
Common symptoms include:
- Eye pain
- Redness
- Blurred vision
- Light sensitivity
- Discharge
Individuals with compromised immune systems, diabetes, or those using corticosteroids face elevated risks. In rare cases, these infections can become life-threatening for immunocompromised patients.
Consumer Protection and Regulatory Oversight
The FDA’s swift response to this recall demonstrates the regulatory body’s commitment to consumer safety in medical devices. However, the incident raises questions about the effectiveness of current quality control protocols in pharmaceutical manufacturing.
The FDA advises consumers who have purchased the recalled lot to:
- Stop using the product immediately
- Return it to the place of purchase for a refund or replacement
- Contact healthcare providers if experiencing any adverse reactions
- Report problems to FDA’s MedWatch Adverse Event Reporting program
Consumers can reach Alcon Laboratories at 1-800-241-5999 between 7:30 AM and 6:00 PM (Central), Monday through Friday.
More Stories
Industry Response and Market Impact
Major retailers have implemented comprehensive recall procedures. Walmart, Target, and other chains have removed affected products from shelves and initiated customer notification protocols. Distributors and retailers received notifications through letters, emails, and phone calls, with instructions to remove and discard remaining stock of the affected lot.
The FDA maintains active oversight of the recall process to ensure proper implementation of safety protocols and consumer protection measures. This incident underscores the need for enhanced sterility testing protocols in preservative-free formulations.
Local pharmacists report increased consumer awareness regarding eye drop safety. “We’re seeing more customers carefully checking lot numbers and expiration dates,” notes James Rodriguez, lead pharmacist at a Fort Worth CVS location.
For additional information about fungal eye infections or to report adverse reactions, consumers can visit the FDA’s official website or contact their healthcare provider.