Cambridge University scientists have made a groundbreaking development with the creation of a solar-powered reactor capable of converting captured carbon dioxide and plastic waste into sustainable fuel while also producing other valuable chemicals. The main output of the reactor is syngas, also known as synthetic gas, which is a combustible fuel that can be directly used as an energy source.
Supplied to the residents of industrialized cities in the 20th century, syngas is more suitable for producing other types of fuels due to its complex nature. The solar-powered device not only addresses carbon dioxide capture but also contributes to recycling by converting plastic waste into fuel and useful chemicals. Pure carbon dioxide (CO2) had been used in previous research on solar fuel cells, which use sunlight to drive chemical reactions for fuel production.
Erwin Reiner and his colleagues at the University of Cambridge are behind the device that can utilize CO2 captured from industrial processes or directly from the air, selectively filtering out other gases. Erwin Reisner’s research group in the Yousuf Hamied Department Of Chemistry at the University of Cambridge conducted the study. Including Reisner, a Professor of Energy and Sustainability, and Sayan Kar, a postdoctoral scientist, are the co-first authors of the Joule article. A significant milestone in the field is marked by this integration of carbon capture and solar fuel technologies. Published in the peer-reviewed journal Joule, the research notes one remarkable aspect: the reactor used carbon dioxide from real-world sources, including industrial exhaust and normal air.
This technology, in the medium term, can contribute to reducing carbon emissions by capturing them from industries such as power plants, cement factories, and biogas plants and converting them into fuel using sunlight and plastic waste. In the long run, it holds promise for direct air capture (DDAC) and the conversion of CO2, leading to the achievement of a circular carbon economy.
Similar Post
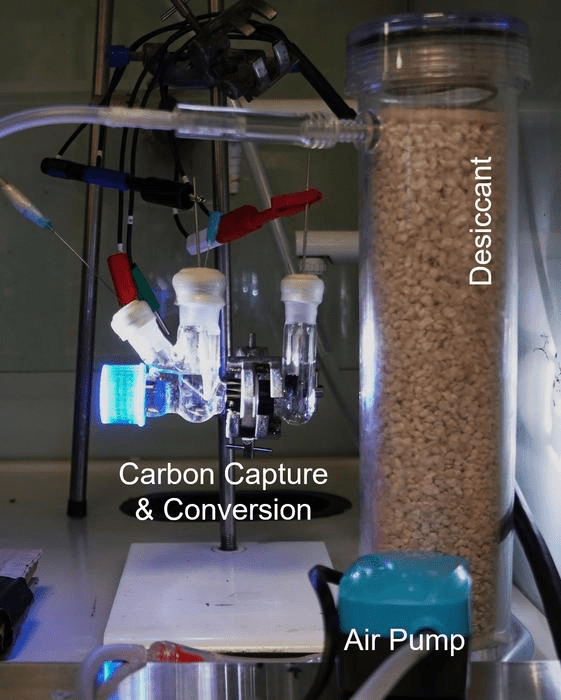
Two compartments make up the device, one filtering air through an alkaline solution that captures CO2 and converts it into syngas, while the other converts PET plastic waste into glycolic acid, a commonly used chemical in cosmetics. As they work in synergy, the integration of the other two compartments is not merely a combination of two separate processes.
It needs to gain electrons for CO2 to transform into syngas. This is achieved by breaking up water molecules, but that process is energy-intensive. Instead, the two compartments function like a battery, with the CO2 side acting as the cathode and the plastic side as the anode, facilitating the transfer of electrons between them.
Currently, while the technology is in the proof-of- concept prototype stage and requires further refinement for large-scale deployment, the team is actively working to improve its efficiency. The researchers envision this technology eliminating the need for new fossil fuels entirely, as it operates in a circular manner, capturing CO2, producing fuel, and subsequently reforming CO2.
This approach offers an alternative to simply capturing and storing CO2 underground. The technology highlights the potential for its utilization rather than permanent burial, thereby providing an avenue for reducing long-term environmental consequences by demonstrating the production of useful products from captured CO2.
Jotheeswari Kothandaraman at the Pacific Northwest National Laboratory previously, was part of the research group that achieved significant success in developing net-zero carbon fuel technologies inspired by photosynthesis, such as artificial leaves capable of converting CO2 into propanol and ethanol.
Jotheeswari believes that the feasibility and carbon footprint of this approach still needs to be assessed through techno-economic and life cycle analyses, as acknowledged by Jotheeswari. Their solar-powered experiments exclusively utilized pure and concentrated CO2 from cylinders, making the utilization of highly diluted CO2 in the air a significant technological challenge.
The team drew inspiration from carbon capture and storage technology to overcome this hurdle, envisioning the capture and utilization of CO2 rather than its mere storage. The process of conversion begins by passing air or flue gas (produced by burning fuel) through an alkaline solution, which traps carbon dioxide while allowing other gases like nitrogen and oxygen to escape.
Further processing is facilitated by this concentration of carbon dioxide, with the alkaline solution capturing CO2 and converting it into syngas on one side while the plastic waste is converted into glycolic acid on the other, as the integrated system contains a photocathode and an anode. A crucial role is played by the plastic waste in donating electrons to the carbon dioxide, leading to the breakdown of plastic into glycolic acid as CO2 is transferred into syngas.
The focus of ongoing research is on enhancing the efficiency, stability, and durability of the system, including optimizing the carbon capture and light absorption processes. A significant advancement in sustainable fuel production and carbon dioxide utilization is presented by the solar-powered reactor, offering potential for a cleaner and more circular energy future.