Researchers from Hunan University, University College London, and the University of Oxford have discovered a novel metal-nitrogen-carbon catalyst for zinc-air batteries (ZABs). It performs better than noble metal catalysts, increasing the usefulness and efficiency of ZAB technology. The scientific publication Nano Research Energy published the breakthrough.
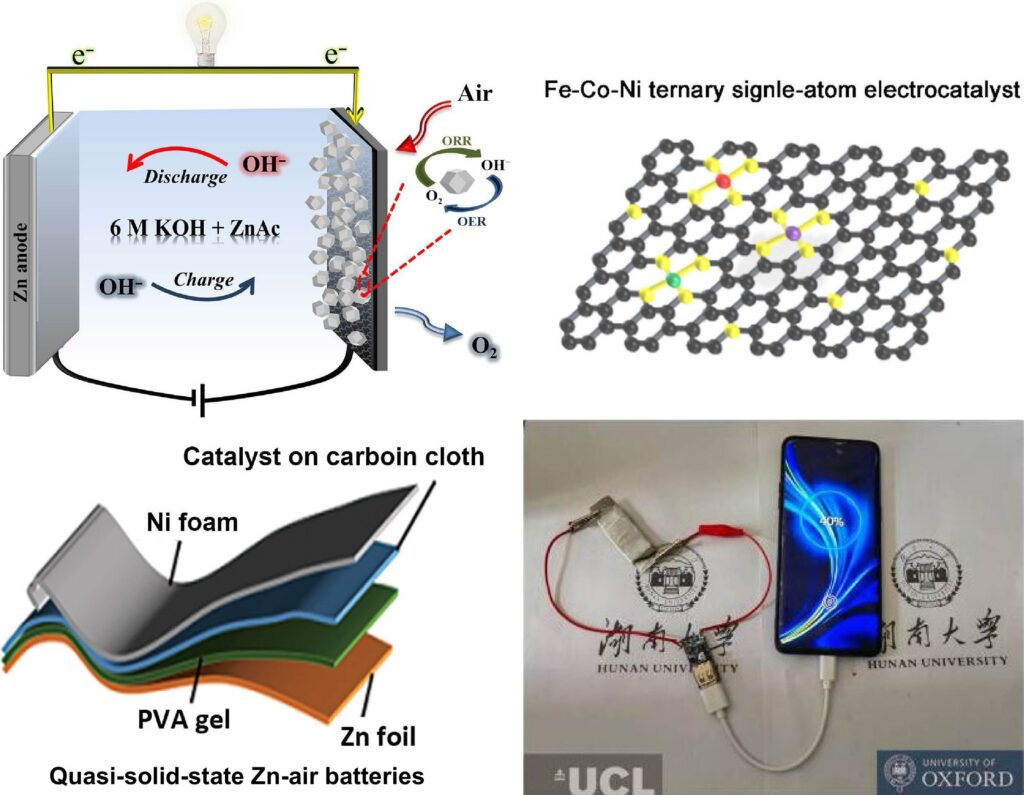
Zinc is oxidized by airborne oxygen in zinc-air batteries. Iron, cobalt, and nickel are the three non-noble metal atoms combined in the newly created catalyst to enhance both charging and discharging operations and increase the cost-effectiveness of ZABs. This catalyst is part of a solid-state ZAB electrolyte consisting of a flexible carbon dot/polyvinyl alcohol (CD/PVA) sheet. The catalyst helps to create a high-performance, flexible battery that can charge a phone, run a fan, and illuminate an LED screen.
Senior author Huanxin Li, a research fellow in the Department of Chemistry at the University of Oxford, stated, “Rechargeable metal-air batteries are promising power sources, especially zinc-air batteries which offer high theoretical energy densities (1084 Wh kg−1), environmental friendliness, and cost-effectiveness.” “Rechargeable ZABs are not only safe and stable but also portable and wearable.”
Similar Posts
In oxygen reduction and evolution processes, the catalyst—known as a ternary Fe-Co-Ni electrocatalyst—performed better than bimetal electrocatalysts and noble metals like ruthenium and platinum. “Developing low-cost and efficient bifunctional non-noble electrocatalysts is crucial to the commercialization of rechargeable ZABs,” said Dr. Li. “Metal-nitrogen-carbon (M-N-C) nanomaterials have attracted particular attention due to their low price, abundant reserves, excellent electrochemical activity, and high stability.”
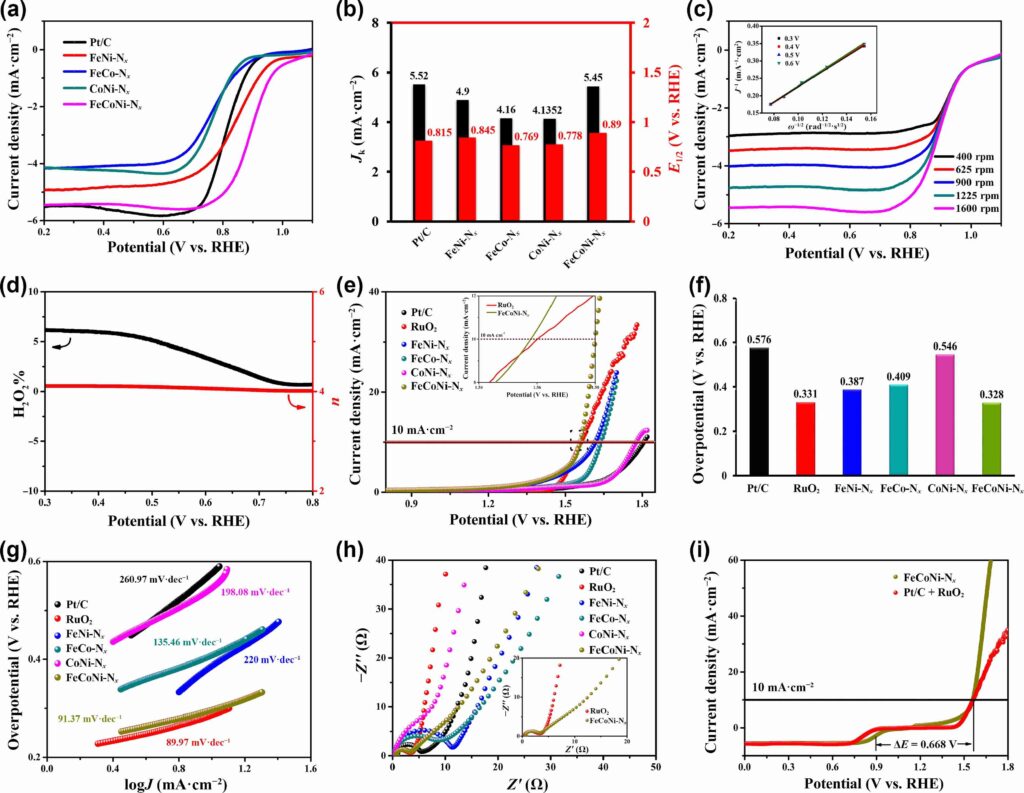
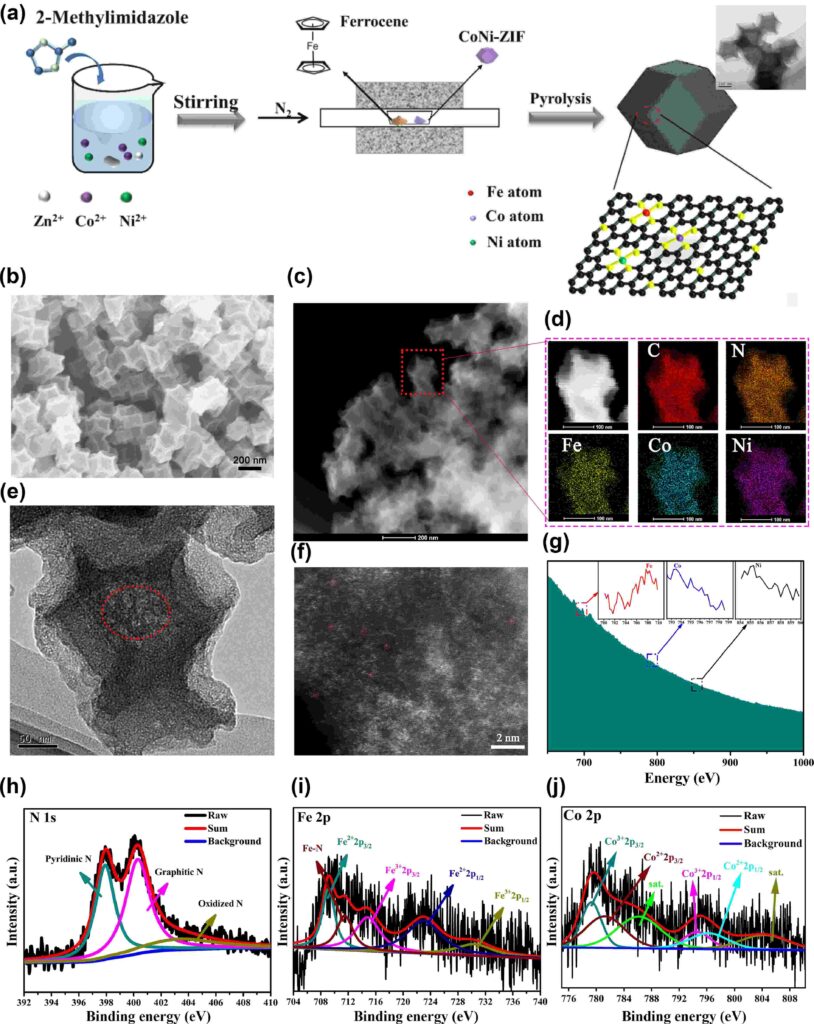
Zeolitic imidazolate frameworks (ZIFs), which are carbon-nitrogen frameworks that encircle and organize each metal atom and bind the catalytic atoms onto porous carbon at high heat, were employed by scientists to develop this electrocatalyst. Power-dispersive X-ray spectroscopy, spherical aberration-corrected high-angle annular dark-field scanning transmission electron microscopy, and electron energy loss spectroscopy were used to establish the distribution of Fe, Co, and Ni atoms.
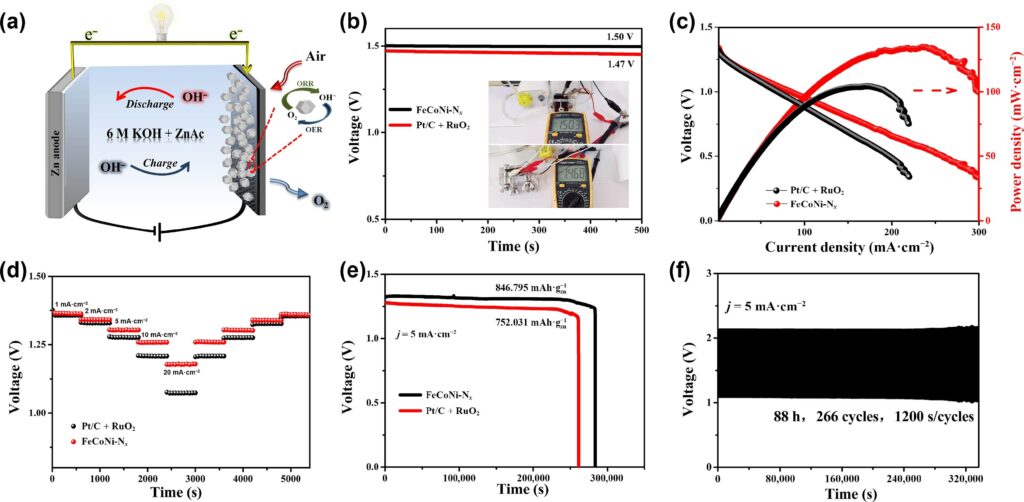
In liquid electrolyte, the team’s rechargeable ZAB produced a power density of 135 mW·cm–2 and a specific capacity of 846.8 mAh·gZn−1. Furthermore, the battery outperformed earlier reported findings for solid-state ZABs with other catalysts, with a power density of 60 mW·cm–2 with the optimized CD/PVA solid-state electrolyte. The researchers hope that more research into novel electrolytes and catalysts for useful, high-performance ZAB technologies will be spurred by their discoveries.