Carbon may be considered a necessary evil. However, the world is struggling today to reduce greenhouse gas emissions. New research by engineers at MIT could lead to rapid improvements in a variety of electrochemical systems. A new design for the electrodes used in these systems is designed by the MIT team. The design increases efficiency of the conversion process. The findings of the study were published in the journal Nature Communications November 13, 2024. The team was led by Kripa Varanasi, Professor of mechanical engineering, MIT. A doctoral student Simon Rufer and three others were part of the study team.
Professor Varanasi says, “The CO2 problem is a big challenge for our times, and we are using all kinds of levers to solve and address this problem.” According to him, it will be essential to find practical ways of removing the gas, either from sources such as power plant emissions, or straight out of the air or the oceans. Once the CO2 has been removed, it has to go somewhere. The team led by Varanasi focused on the electrochemical conversion of CO2 to ethylene, a widely used chemical that can be made into a variety of plastics as well as fuels. These fuels today are made from petroleum. The approach could also be applied to producing other high-value chemical products, including methane, methanol, carbon monoxide and others. At present, the cost of ethylene is $1,000 per ton. The goal is to meet or beat that price.
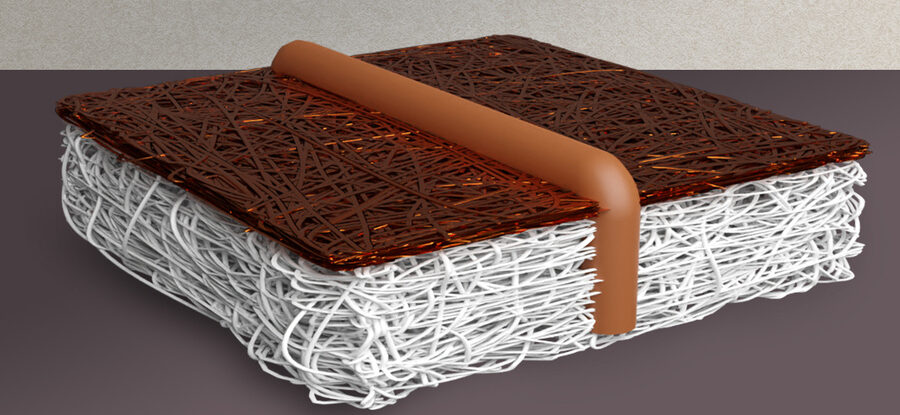
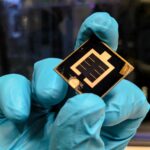
The solution, devised by Varanasi and Rufer, is elegant in its simplicity. The researchers wove a series of conductive copper wires through the very thin sheet of the PTFE (essentially Teflon). Varanasi says, “This work really addressed this challenge, as we can now get both conductivity and hydrophobicity.” The team of Varanasi produced a sheet 10 times larger in area and demonstrated its effective performance.
The team found that conductivity dropped off dramatically with size, which would mean much more energy, and thus cost, would be needed to drive the reaction. The larger sizes produced more unwanted chemical byproducts besides the intended ethylene. The MIT team developed a model which captures the spatial variability in voltage. The model enabled the optimal spacing for conductive wires to counteract the drop off in conductivity.
Similar Posts
According to Rufer, the material was split into a bunch of little subsegments, each of which is effectively a smaller electrode. Rufer says, “And we’ve seen, small electrodes can work really well.” The researchers ran a test electrode for 75 hours continuously, with little change in performance. The MIT system, according to Rufer, is the first PTEE-based electrode which has gone beyond the lab scale in order of 5 centimeters or smaller. He adds that the weaving process for incorporating the wire can be easily integrated into existing manufacturing processes, even in a large-scale roll-to-roll process. He adds, “Our approach is very powerful because it doesn’t have anything to do with the actual catalyst being used.”
Varanasi says, “Given that we will need to process gigatons of CO2 annually to combat the CO2 challenge, we really need to think about solutions that can scale.” According to Varanasi, this insight enables us to identify critical bottlenecks and develop innovative approaches that can make a meaningful impact in solving the problem. The MIT hierarchically conductive electrode is a result of such thinking.
The team included MIT graduate students Michael Nietzsche and Sanjay Garimella, as well as Jack Lake, Ph.D ’23. Shell supported the research work of the Varanasi team through the MIT Energy Initiative.
In short, a new electrode design made by MIT engineers led by Professor Kripa Varanasi boosts the efficiency of electrochemical reactions that turn carbon dioxide into ethylene and other products. The findings of the MIT study were published in the journal Nature Communications November 13, 2024. This finding is a welcome breakthrough to a solution to the challenge of greenhouse gas emissions.