Chemical engineers at MIT have developed a hybrid catalyst that converts methane into polymers at room temperature and atmospheric pressure. The research appears in Nature Catalysis.
Methane contributes about 15% to global temperature increases, trapping more atmospheric heat than carbon dioxide due to its molecular structure. Current conversion methods require high temperatures and pressures, making them energy-intensive and expensive.
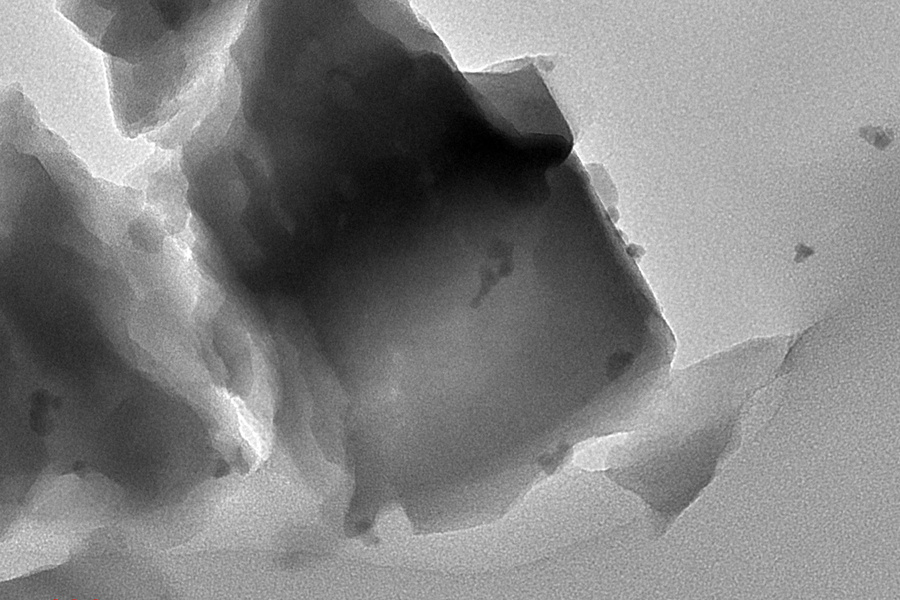
The new catalyst combines iron-modified aluminum silicate (a zeolite) with alcohol oxidase enzyme in a two-step process. The zeolite converts methane to methanol, while the enzyme transforms methanol into formaldehyde with over 90% selectivity. A notable feature is the catalyst’s self-sustaining nature – it generates hydrogen peroxide as a byproduct, which feeds back into the zeolite for methane-to-methanol conversion.
“What to do with methane has been a longstanding problem. It’s a source of carbon, and we want to keep it out of the atmosphere but also turn it into something useful,” says Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT and senior study author.
The formaldehyde produced combines with urea to create urea-formaldehyde polymers, used in particle board, textiles, and various consumer products. The research team envisions applying the catalyst in natural gas pipelines to seal methane leaks and as surface coatings to capture methane emissions.
More Stories
Professor Damien Debecker from the University of Louvain, Belgium, who wasn’t involved in the study, notes: “Combining these two families of catalysts is challenging, as they tend to operate in rather distinct operation conditions. By unlocking this constraint and mastering the art of chemo-enzymatic cooperation, hybrid catalysis becomes key-enabling: It opens new perspectives to run complex reaction systems in an intensified way.”
The U.S. Department of Energy funded this research, which utilized MIT.nano’s characterization facilities. The research team includes Daniel Lundberg PhD ’24, postdoc Jimin Kim, former postdoc Yu-Ming Tu, and postdoc Cody Ritt.
The catalyst particles are suspended in water, which can absorb methane from the surrounding air. “Other systems operate at high temperature and high pressure, and they use hydrogen peroxide, which is an expensive chemical, to drive methane oxidation. But our enzyme produces hydrogen peroxide from oxygen, so I think our system could be very cost-effective and scalable,” says Kim.Strano’s lab continues research on catalysts that could remove atmospheric carbon dioxide and combine it with nitrate to produce urea, potentially creating a closed-loop system for urea-formaldehyde production.