A new cancer vaccine for advanced melanoma patients is being fast-tracked through the NHS in England, offering fresh hope to those battling this serious form of skin cancer.
The vaccine, called iSCIB1+ (Immunobody), developed by UK-based Scancell, works differently from preventative vaccines. It helps the body’s immune system better recognize, attack and “remember” cancer cells, potentially stopping the disease from returning.
NHS England has expanded its world-first Cancer Vaccine Launch Pad (CVLP) program to include this melanoma vaccine trial. Seven hospital sites are initially registered to offer the treatment, with patient referrals expected to begin next month.
“Skin cancer can have a devastating impact and we know that cancer vaccines have the potential to revolutionize cancer care for patients in this country and across the world – and to save more lives,” said NHS National Cancer Director Professor Peter Johnson.
Why This Matters
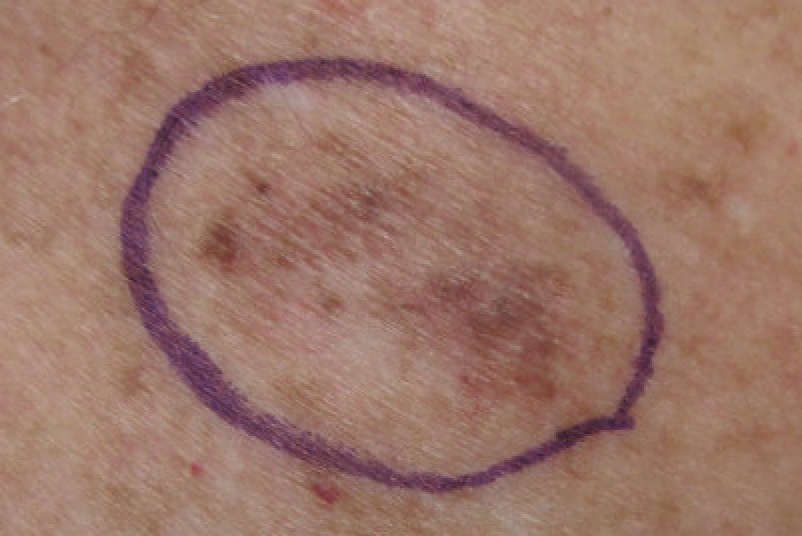
Melanoma is the fifth most common cancer in the UK, accounting for about 4% of all new cancer cases. Data from Cancer Research UK shows cases rose by a third between 2009-2019.
While about half of melanoma patients respond well to standard immunotherapy treatments, those who don’t respond face a higher risk of their cancer progressing. This vaccine aims to improve those odds.
The phase II clinical trial, known as SCOPE, is coordinated by the Southampton Clinical Trials Unit. Researchers hope to recruit dozens of patients by October.
How It Works
The iSCIB1+ vaccine targets specific biomarkers found on melanoma tumors. These biomarkers act like flags that alert the immune system. When the vaccine is administered, it triggers T-cells (a type of immune cell) to seek out and destroy cancer cells displaying these markers.
Unlike personalized cancer vaccines that are tailored to each patient’s specific tumor, this is an “off-the-shelf” treatment designed to work for eligible patients with advanced melanoma.
To determine eligibility, patients with advanced melanoma who haven’t yet received treatment will first need a blood test to check their tissue type (HLA type). This test examines genes that control how the immune system works, which vary from person to person.
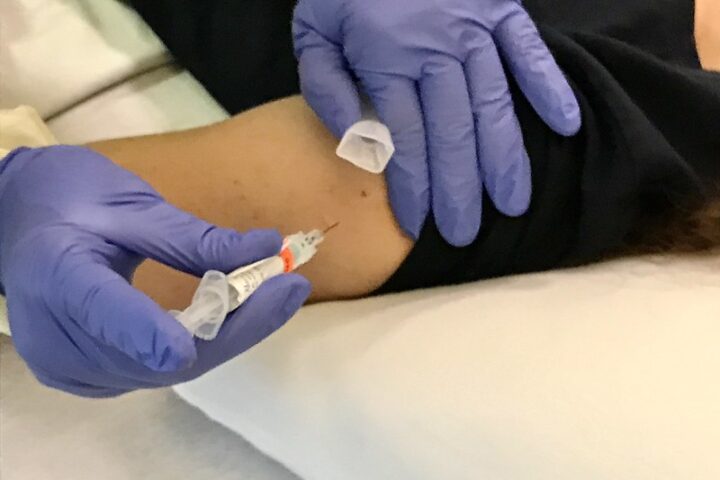
The vaccine is administered via needle-free injection, either into the skin (intradermal) or muscle (intramuscular), and treatment can last up to two years.
Similar Posts
Real Patient Experience
Paul Thomas, a 63-year-old grandfather of four from Hampshire, joined the SCOPE trial after being diagnosed with advanced skin cancer in 2017. His cancer had repeatedly returned following treatment.
“Since I’ve been on it, my tumors have all shrunk. Every time I go for a scan they seem to be shrinking, which is really exciting,” said Thomas. “I’m really hoping for total eradication of my cancer, as opposed to being put in remission and I’m feeling optimistic.”
Expert Perspectives
Dr. Nermeen Varawalla, Chief Medical Officer at Scancell, pointed to promising early results: “Recent clinical data has demonstrated that our potent, tumor-targeted ‘off-the-shelf’ cancer vaccine delivers strong efficacy, with the potential for meaningful long-term survival benefits in patients with advanced metastatic melanoma.”
Susanna Daniels, CEO of Melanoma Focus, welcomed the development: “Melanoma skin cancer can be deadly and it’s sadly on the rise in the UK. As well as continuing to call for urgent action on prevention, we’re delighted to see progress in innovative treatments.”
The charity offers a Melanoma TrialFinder to help eligible patients locate trial centers and discuss options with their medical teams.
Prime Minister Keir Starmer has also supported the initiative: “This kind of innovation is nothing short of life-saving and I want to see more of these world-leading treatments being developed in the UK.”

The Bigger Picture
The Cancer Vaccine Launch Pad aims to provide 10,000 patients in England with personalized cancer treatments by 2030. It has already begun helping thousands of patients access trials of a personalized vaccine against bowel cancer, with more than 350 patients fast-tracked for consideration.
Full data from the ongoing SCOPE trial is expected in mid and late 2025. These results will be crucial in determining the next steps for this promising treatment approach.
This melanoma vaccine trial represents part of a broader trend in cancer research toward harnessing the body’s own immune system to fight cancer, offering new options for patients who may not respond to existing treatments.