On October 17, 2023, Chalmers University of Technology dropped some exciting news: a fresh, green approach to battery recycling. Léa Rouquette, a bright PhD mind at Chalmers, shared a first-of-its-kind achievement: separating a heap of lithium using plant-based oxalic acid and waving goodbye to all the aluminium. Why the buzz? Every battery packs aluminium, and getting it out without losing other metals is the trick.

“So far, no one has managed to find exactly the right conditions for separating this much lithium using oxalic acid, whilst also removing all the aluminium. Since all batteries contain aluminium, we need to be able to remove it without losing the other metals.”
Léa Rouquette, PhD student at the Department of Chemistry and Chemical Engineering at Chalmers.
Enter oxalic acid, nature’s gift from plants like rhubarb and spinach, now a hero for dissolving old car battery cells. Rouquette teamed up with research ace Martina Petranikova, and together they showcased a neat trick: turn battery innards into a sleek black powder, then let oxalic acid work its magic. It might sound as easy as making your morning brew, but trust us, it’s science gold. Tweaking the mix with the right temperature, concentration, and timing, they’ve brewed an eco-friendly oxalic acid formula.
Similar Posts
Martina Petranikova, Chalmers’ Associate Professor, voiced out loud what many were thinking: we need green alternatives in recycling. Today’s recycling world faces a sticky problem: getting rid of pesky leftovers like aluminium. But Chalmers’ latest might just be the answer we’ve been waiting for. Old-school methods? They’d melt all metals in an EV battery, clean up the mess, and then salvage the good stuff. But here’s the kicker: that old route often left lithium, our star player, behind.
Chalmers’ new playbook? Go for lithium and aluminium first. Less waste, more win. Next up, filter out the black mix. What you get is aluminium and lithium in the liquid, with other metals chilling as solids. Rouquette’s take? With metals being so unique, splitting them up is a breeze. She’s stoked about this new path in battery recycling and thinks it’s worth every bit of the deep dive. Petranikova’s dreaming big, hoping industries will jump on this green train soon.

“We need alternatives to inorganic chemicals. One of the biggest bottlenecks in today’s processes is removing residual materials like aluminium. This is an innovative method that can offer the recycling industry new alternatives and help solve problems that hinder development.”
Martina Petranikova, Associate Professor at the Department of Chemistry and Chemical Engineering at Chalmers
Chalmers University? They’re no rookies. With Petranikova’s squad leading the charge, they’ve been pushing the boundaries of lithium-ion battery recycling. They’re in good company, teaming up with big names like Volvo Cars and Northvolt to supercharge electric car battery recycling. For those who love the nitty-gritty, their research, “Complete and selective recovery of lithium from EV lithium-ion batteries: Modeling and optimization using oxalic acid as a leaching agent,” is up for grabs in “Separation and Purification Technology.”
Behind this genius? Léa Rouquette, Martina Petranikova, and Nathália Vieceli, the Chalmers’ trio. Shoutout to the Swedish Energy Agency, BASE Batteries Sweden, and Vinnova for funding this game-changer. And those old electric car batteries from Volvo Cars? Recycled to perfection by Stena Recycling and Akkuser Oy.
“Since the metals have very different properties, we don’t think it’ll be hard to separate them. Our method is a promising new route for battery recycling – a route that definitely warrants further exploration.”
Léa Rouquette, PhD student at the Department of Chemistry and Chemical Engineering at Chalmers.
Diving into “Separation and Purification Technology,” oxalic acid is the MVP for selective leaching. They didn’t just wing it; they designed experiments to nail the perfect mix. And the scoreboard? A solid 98% lithium recovery, with aluminium joining the party at 100%. But here’s the cool part: cobalt and nickel barely made a dent at 0.5%, and manganese was just chilling at 1.5%. With batteries being the heart of our green future, efficient recycling isn’t just a want; it’s a need.
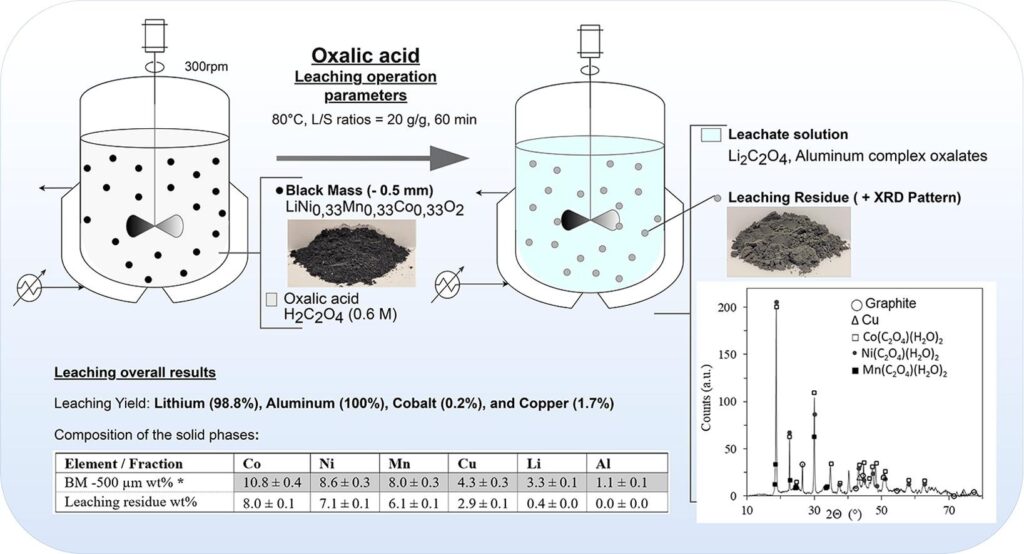
Their deep dive into early lithium recovery using oxalic acid? Pure genius, leveraging the quirky nature of metal oxalates. While nickel, cobalt, and manganese played hard to get, lithium was all in. The winning formula? A cozy 60°C, a patient 60 minutes, and a dash of 0.6M oxalic acid. The result? A jaw-dropping 98.8% lithium score, setting a new bar in lithium recovery. And that aluminium dissolve? That’s a first, and it’s got the recycling world buzzing.
Their insights lead us to a roadmap to lithium gold, something the industry’s been chasing. Their method’s potential could flip the battery recycling world on its head. With EVs zooming into the future, green innovations like these are the fuel we need. With researchers, industries, and green-thinkers joining forces, our green dreams are revving up to hit the fast lane.















