A new study published in Nature Geoscience proposes that liquid carbon dioxide, alongside water, may have shaped Mars’ ancient surface features and mineral composition.
The perspective article, led by Michael Hecht, principal investigator of the MOXIE instrument aboard the NASA Mars Rover Perseverance and research scientist at MIT’s Haystack Observatory and former associate director, challenges the long-held assumption that water alone created Mars’ dry river channels and lake beds. “Understanding how sufficient liquid water was able to flow on early Mars to explain the morphology and mineralogy we see today is probably the greatest unsettled question of Mars science,” states Hecht.
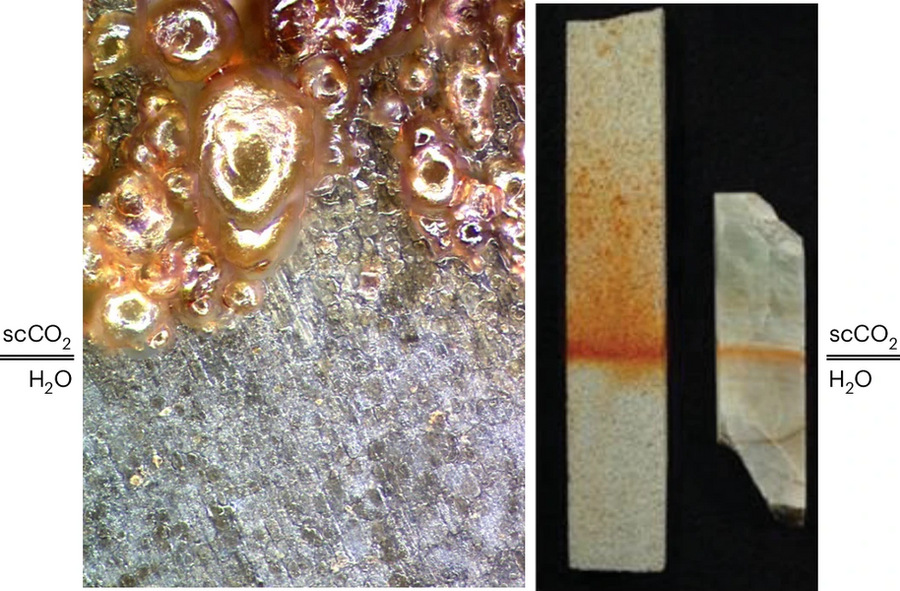
The research draws parallels from Earth’s carbon sequestration studies, where mineral alterations occur in liquid CO2 environments, sometimes showing rapid alteration processes. These findings suggest similar processes could have occurred on Mars, potentially explaining the presence of carbonates, phyllosilicates, and sulfates observed on the planet‘s surface.
The study outlines three scenarios for liquid CO2’s presence on Mars: stable surface liquid, basal melting under CO2 ice, and subsurface reservoirs. The feasibility of each scenario depends on the actual inventory of CO2 at the time and surface temperatures.
More Stories
However, the researchers acknowledge key differences between Earth’s carbon sequestration conditions – where liquid CO2 exists above room temperature at high pressures – and Mars’ colder, lower-pressure environment. They emphasize the need for laboratory testing under Mars-like conditions to verify these chemical reactions.
“There is likely no one right answer, and we are merely suggesting another possible piece of the puzzle,” Hecht adds, maintaining scientific caution while presenting this alternative hypothesis.
This research opens new avenues for understanding Mars’ geological history, suggesting a more complex interplay between different liquids in shaping the Red Planet’s surface features and mineral deposits.