A major breakthrough in Huntington’s disease research has revealed why this devastating genetic condition typically strikes people in their prime, decades after birth. Scientists have discovered that the disease-causing mutation isn’t immediately harmful – instead, it grows quietly over time until reaching a dangerous tipping point.
The research, published in Cell by scientists at Harvard Medical School, the Broad Institute, and McLean Hospital, solves a decades-old puzzle about this fatal brain disorder.
“The conundrum in our field has been: Why do you have a genetic disorder that manifests later in life if the gene is present at conception?” explains Dr. Mark Mehler, director of the Institute for Brain Disorders and Neural Regeneration at Albert Einstein College of Medicine, who wasn’t involved in the study. He calls it a “landmark” discovery.
The Secret Life of a Mutation
The story begins with a small genetic change – a three-letter DNA sequence (CAG) that repeats too many times. While most people have 15 to 35 repeats of this sequence, those with Huntington’s have 40 or more.
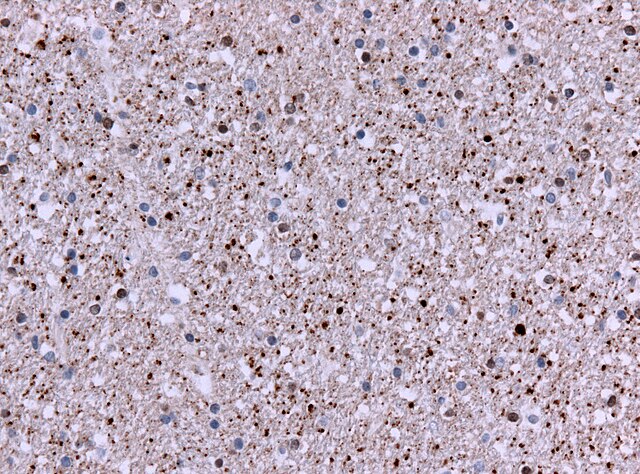
The research team studied brain tissue from 53 people with Huntington’s and 50 without it, analyzing half a million cells. They found something unexpected: the mutation starts small but grows over decades, over time.
In the brain‘s striatal projection neurons – cells that control movement and thinking – these CAG repeats gradually increase. The process is slow at first, with less than one new repeat added each year during childhood and early adulthood. But when the number reaches about 80 repeats, usually after several decades, the growth speeds up dramatically.
The Critical Threshold
The most surprising finding is that these expanding repeats don’t immediately harm brain cells. Even up to 150 repeats, the neurons remain healthy. But cross that threshold, and the cells quickly sicken and die, leading to the disease’s devastating symptoms – involuntary movements, personality changes, , impaired judgment, and problems with thinking and behavior.
“It’s only when the repeat becomes extremely long that it begins to cause harm,” says Steve McCarroll, co-senior author and professor at Harvard Medical School.
Similar Posts
New Hope for Treatment
This discovery changes how scientists think about treating Huntington’s. Previous treatments tried to reduce the activity of the mutated gene, but these attempts struggled in clinical trials. The new research suggests why – these treatments might only help the few cells that have already crossed the dangerous threshold.
Instead, researchers now believe that stopping or slowing the growth of CAG repeats might be more effective. This could delay or even prevent cells from reaching the toxic threshold, potentially pushing back the onset of symptoms or preventing them entirely.
“It’s going to take much scientific work by many people to get to treatments that slow the expansion of DNA repeats,” McCarroll notes. “But we’re hopeful that understanding this as the central disease-driving process leads to deep focus and new options.”
A Legacy of Knowledge
The research wouldn’t have been possible without the generosity of brain tissue donors and their families. “Our gratitude is with the families that chose to do something that is very difficult to do,” says co-senior author Sabina Berretta. “This would not have been possible without the altruism of many brain donors who have left a legacy of knowledge that will last and benefit many other people.”
The findings offer hope not just for Huntington’s but potentially for understanding other genetic disorders involving DNA repeats, including fragile X syndrome and myotonic dystrophy. As researchers work to develop new treatments based on these insights, they’re racing against time to help the approximately 41,000 Americans affected by Huntington’s disease.