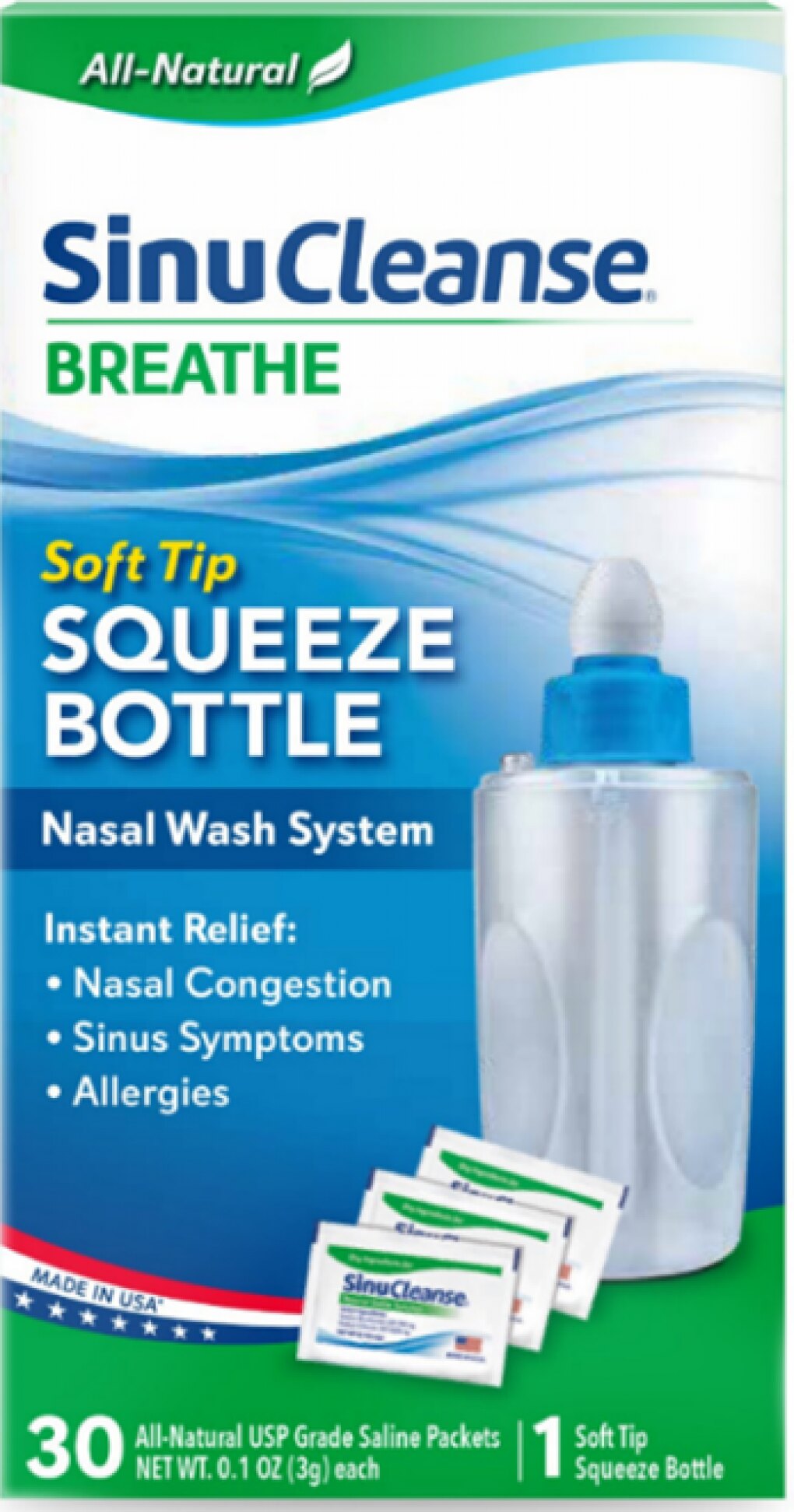
February 26, 2025 – Ascent Consumer Products Inc. has issued a voluntary nationwide recall of one lot of their SinuCleanse Soft Tip Squeeze Bottle Nasal Wash System after testing confirmed contamination with Staphylococcus aureus bacteria.
The affected products were distributed across the United States in January 2025 through both retail stores and online sellers. The recall specifically targets lot number 024122661A1 with an expiration date of December 31, 2027.

Health officials emphasize the serious nature of this contamination. Staphylococcus aureus contamination in nasal wash products is particularly concerning because the bacteria has direct access to sensitive nasal passages, which can lead to serious infections.
The FDA announcement on February 25 detailed the potential health risks, which include blood infections, endocarditis (infection of the heart’s inner lining), bone and joint infections, splenic abscesses, meningitis, and bacterial sinusitis. These conditions can be life-threatening if not treated promptly.

The SinuCleanse Soft Tip Squeeze Bottle Nasal Wash System is designed to help relieve symptoms associated with sinusitis, cold, flu, or allergies by washing out the nasal passages. Each recalled unit consists of a carton containing a squeeze bottle and 30 saline packets.
“The risk is highest for users whose nasal mucosa may be compromised due to inflammation or mechanical injuries caused by nasal irrigation,” according to the company’s official recall notice. Despite the serious nature of the recall, Ascent Consumer Products has reported that no adverse events related to this contamination have been confirmed as of February 25.

Consumers in possession of the affected product should:
- Stop using the product immediately
- Either return it to the place of purchase or discard it
- Contact their healthcare provider if they experience any issues after using the product

Retailers and distributors have been instructed to remove the affected lot from inventory and cease distribution immediately. The company is contacting all distributors and customers directly through electronic mail.
Similar Posts
The recall is being conducted with full knowledge of the U.S. Food and Drug Administration. Consumers with questions regarding the recall can contact Ascent Consumer Products Inc. by email at [email protected], Monday through Friday from 9am to 5pm Eastern Time.
Any adverse reactions experienced with this product should be reported to the FDA’s MedWatch Adverse Event Reporting program.
This recall highlights the importance of rigorous testing and quality control for products that come into contact with sensitive areas of the body. Health experts recommend checking all medical devices and products for recall notices regularly, particularly those used for nasal or respiratory care.