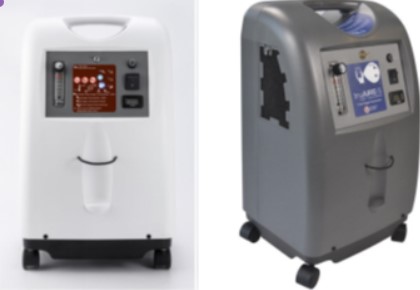
A critical safety recall has been issued for TruAire-5 oxygen concentrators after reports of devices catching fire during use. Jiangsu Jumao X-Care Medical Equipment Co. Ltd., the manufacturer, has recalled all units within the serial number range JA2311000001 to JA2312000740 of their JMC5A Ni/TruAire-5 model.
The U.S. Food and Drug Administration has classified this as a Class I recall – their most serious designation – indicating these devices may cause serious injury or death. While no injuries or deaths have been reported so far, the potential for the devices to melt or catch fire poses significant risks to users who depend on these machines for supplemental oxygen.
The TruAire-5 O2 Concentrator (Model O2C5L) works by separating nitrogen from room air using a molecular sieve to provide supplemental oxygen for patients with respiratory disorders. While not designed for life support, these devices play an important role in managing respiratory conditions at home.
The manufacturer sent urgent recall notices to affected customers on November 26, 2024, with specific instructions for immediate action. Customers must stop using these devices immediately and return them to Compass Health Brands, the distributor handling the recall process.
The exact cause of the fires and melting remains under investigation. This situation is particularly concerning given that oxygen concentrators typically run continuously in homes where people may be sleeping or unable to respond quickly to equipment failures.
Similar Posts
For safety reasons, customers must take three key steps:
1. Stop using the device immediately
2. Remove it from service
3. Contact Compass Health Brands for return instructions
U.S. customers with questions about this recall can reach Compass Health Brands at 1-800-376-7263 x444. Healthcare providers and consumers who experience problems with these devices should report them through the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The FDA’s tracking of these devices through their Unique Device Identifier (UDI) system will help monitor the recall’s progress and potentially identify the root cause more quickly. This system allows for precise tracking of medical devices from manufacturing through distribution to patient use, enabling faster response to safety issues.
Affected customers should also complete and return the medical device recall acknowledgment form to Compass Health Brands within 15 calendar days, even if they no longer have the device or never purchased it.