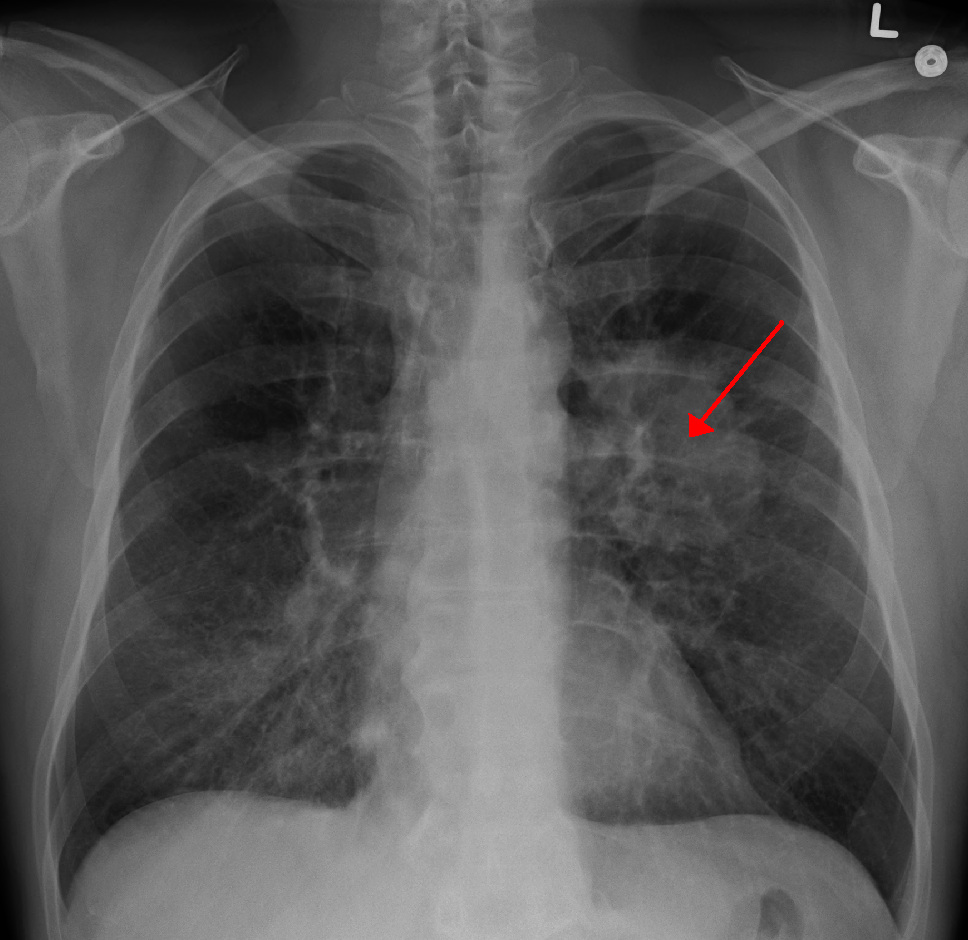
A new cancer treatment just received special recognition from the FDA, marking an important step forward for patients with an aggressive type of lung cancer. The treatment, developed by Ariceum Therapeutics, targets specific receptors on cancer cells called SSTR2, offering a more precise way to deliver treatment to cancer cells.
The FDA gave this treatment what’s called “Orphan Drug” status. This special status helps speed up the development of treatments for rare diseases by giving companies extra support and protection. It’s particularly important because this type of lung cancer – called Small Cell Lung Cancer (SCLC) – is especially hard to treat.
“This recognition shows the treatment’s potential to help patients who have very few options right now,” says Manfred Rüdiger, who leads Ariceum Therapeutics.
The numbers show why this matters. Right now, when doctors diagnose this type of lung cancer, it has usually already spread in two out of three patients. Only 5-10 out of every 100 patients survive for five years after diagnosis. These low survival rates show why new treatments are urgently needed.
What makes this treatment different is how it works. It’s being developed as what doctors call a ‘theranostic pair‘ – combining diagnosis and treatment together. The diagnostic tool (called 68Ga-SSO120) helps identify which patients might benefit from the treatment (called 225Ac-satoreotide), allowing for more personalized cancer care.
Recent tests in laboratories have shown good results. The company reported in October 2024 that their treatment worked better than similar existing options. The next big step comes in early 2025 when doctors will begin testing it in patients through carefully controlled clinical trials called SANTANA-225.
The FDA’s special status gives the company seven years where they would be the only ones allowed to sell this treatment if it gets final approval. This protection helps ensure companies invest time and money in developing treatments for rare diseases that might otherwise be ignored.
For families affected by this type of lung cancer, this development means a potential new option might be available in the future. While the treatment still needs to prove itself in clinical trials, the FDA’s support helps speed up this process.