Boots has issued an urgent recall for certain packs of its own-brand paracetamol due to a serious packaging error that could pose risks to patients.
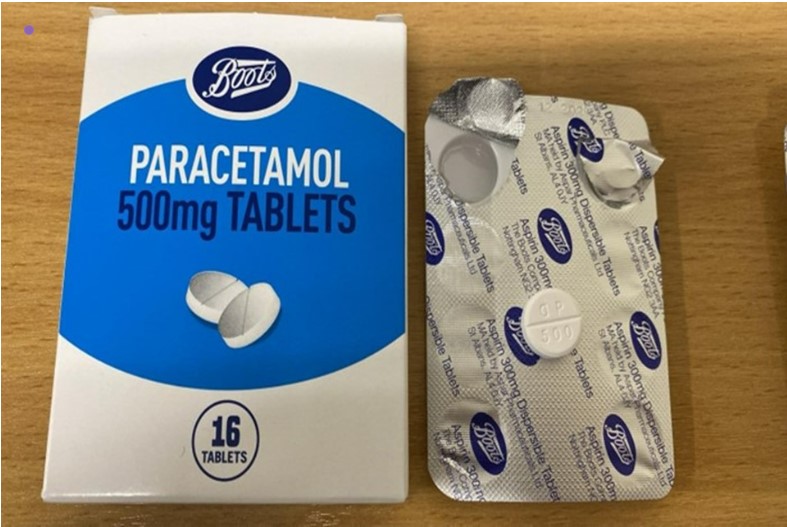
The UK Medicines and Healthcare products Regulatory Agency (MHRA) announced on March 4 that specific batches of Boots Paracetamol 500mg Tablets are being recalled because the foil blister inside the carton incorrectly states ‘Aspirin 300mg Dispersible Tablets’ instead of ‘Paracetamol 500mg Tablets’.
While the tablets themselves are confirmed to be paracetamol, the mislabeling could lead to confusion and potential health risks, especially for those with aspirin allergies or other medical conditions where aspirin is not recommended.
Affected Products
The recall specifically affects Boots Paracetamol 500mg Tablets 16s with:
- Item code: 81-99-922
- Batch number: 241005
- Expiry date: December 2029
119,964 packs are affected by this recall, according to the MHRA.
Dr. Stephanie Millican, MHRA Deputy Director Benefit Risk Evaluation, emphasized the importance of checking packaging: “Patient safety is always our priority. It is vitally important that you check the packaging of your Boots Paracetamol 500mg Tablets 16s, and if the batch number is 241005, you should stop using the product and return it to a Boots store for a full refund.”
Similar Posts
What to Do
Customers who have purchased the affected product are advised to:
- Stop using the product immediately
- Return it to any Boots store for a full refund (no receipt required)
- Not keep the affected packs at home, even if aware of the error, as this could lead to confusion and incorrect dosing
Those who have already taken tablets from the affected batch should seek advice from a healthcare professional if they have concerns or have experienced any side effects.
“If you have purchased this product for someone else, you should inform them as soon as possible,” the MHRA advised.
Investigation Underway
Boots and the supplier, Aspar Pharmaceuticals Limited, have confirmed the tablets in the blister packs are indeed paracetamol 500mg and not aspirin. Both companies are conducting a full investigation into how the packaging error occurred.
This type of recall is classified as a Class 2 Medicines Recall by the MHRA.

According to the NHS, both paracetamol and aspirin are common painkillers but work differently in the body. Paracetamol may be better for headaches, toothache, sprains, and stomach ache, while aspirin may be better for period pain or migraines. Aspirin works by stopping the body from making compounds called prostaglandins, which helps lower pain and reduce swelling and fever.
The MHRA encourages patients who experience any suspected adverse reactions to report them via the MHRA Yellow Card Scheme, which monitors the safety of medicines in the UK.
FAQs
How do I check if my Boots paracetamol is affected by the recall?
Look for the batch number 241005 on the bottom of the box. The affected products are Boots Paracetamol 500mg Tablets 16s with item code 81-99-922 and expiry date December 2029.
What should I do if I have the recalled paracetamol?
Stop using the product immediately and return it to any Boots store for a full refund. You don’t need a receipt to get the refund.
Are the tablets themselves dangerous?
The tablets are confirmed to be paracetamol 500mg, not aspirin. The issue is with the incorrect labeling on the foil blister pack, which could lead to confusion about what medication you’re taking.
What if I’ve already taken tablets from the affected pack?
If you’ve taken tablets and are concerned or have experienced any side effects, seek advice from a healthcare professional. You can also report any suspected adverse reactions via the MHRA’s Yellow Card Scheme.
Why is this mislabeling a health concern?
The mislabeling could cause confusion about which medication you’re taking. This is particularly concerning for people who have allergies to aspirin or medical conditions where aspirin is contraindicated. It could also lead to incorrect dosing.
How many packs are affected by this recall?
According to the MHRA, 119,964 packs of Boots Paracetamol 500mg Tablets are affected by this recall.