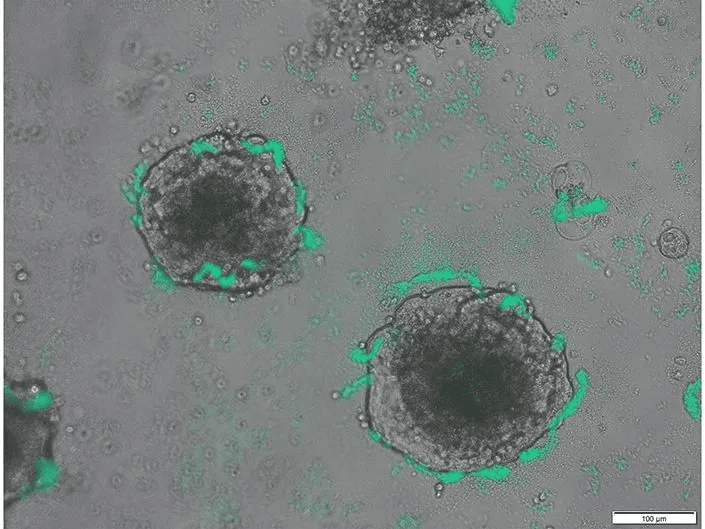
Scientists from the University of California San Diego, in collaboration with their Australian colleagues, have engineered a bacterium with the capability to detect the presence of tumor DNA in a live organism. This innovation, which has successfully detected cancer in the colons of mice, was detailed in the journal Science on August 11th, 2023. It holds the potential to lay the foundation for advanced biosensors that might identify a range of infections and other diseases.
Historically, bacteria have been modified to perform various diagnostic and therapeutic functions. However, their capability was limited, lacking the precision to identify specific DNA sequences and mutations outside of cells. Addressing this limitation, the new “Cellular Assay for Targeted CRISPR-discriminated Horizontal gene transfer,” aptly termed “CATCH,” was conceptualized.
Jeff Hasty, the scientific team leader and a professor at the UC San Diego School of Biological Sciences and Jacobs School of Engineering, shared his insights, stating, “As we embarked on this project four years ago, the idea of using bacteria as a sensor for mammalian DNA seemed ambitious. Yet, the potential for detecting gastrointestinal cancers and precancerous lesions using this method is a promising clinical application.”
It’s well-documented that tumors release their DNA into their surrounding environments. While many existing technologies can analyze purified DNA in lab settings, they often fall short in detecting DNA at its source. Utilizing the CATCH technology, the researchers employed CRISPR to assess DNA sequences on a genomic level, comparing them against known cancer sequences. Rob Cooper, the study’s co-first author and a scientist at UC San Diego’s Synthetic Biology Institute, elaborated, “Many bacteria exhibit the ability to absorb DNA from their environment, a phenomenon known as natural competence.”
The research was further propelled by the collaboration between Australian doctors Dan Worthley, Hasty, and Cooper, focusing on the concept of natural competence in bacteria concerning colorectal cancer, a leading cause of cancer-related deaths in the U.S. The team’s vision revolved around the potential of engineering bacteria, already abundant in the colon, into novel biosensors. Their attention was particularly drawn to Acinetobacter baylyi, a bacterium wherein Cooper discerned the essential components for DNA uptake and subsequent CRISPR analysis.
Similar Post
Dr. Dan Worthley, a gastroenterologist and cancer researcher at the Colonoscopy Clinic in Brisbane, Australia, expressed, “Recognizing that cell-free DNA can act as a signal, our aim was to design bacteria that would resonate with tumor DNA, facilitating real-time disease detection.”
Building upon established concepts, the research delved into horizontal gene transfer, a method organisms use to exchange genetic material distinct from traditional inheritance. While this transfer is commonly observed between bacteria, the team’s achievement lies in extending this principle from mammalian tumors and human cells to bacteria.
Dr. Worthley optimistically concluded, “We envision a future where colorectal cancer is no longer fatal. We are hopeful that our endeavors will resonate with bioengineers, scientists, and eventually, medical practitioners.”
This pivotal research was funded by the National Institutes of Health (grant R01CA241728) and a National Health and Medical Research Council (Australia) ideas grant (2020555). The esteemed co-authors of the study include Robert Cooper, Josephine Wright, Jia Ng, Jarrad Goyne, Nobumi Suzuki, Young Lee, Mari Ichinose, Georgette Radford, Feargal Ryan, Shalni Kumar, Elaine Thomas, Laura Vrbanac, Rob Knight, Susan Woods, Daniel Worthley, and Jeff Hasty.