A Washington-based company has recalled a dietary supplement that could pose serious health risks to people with milk allergies.
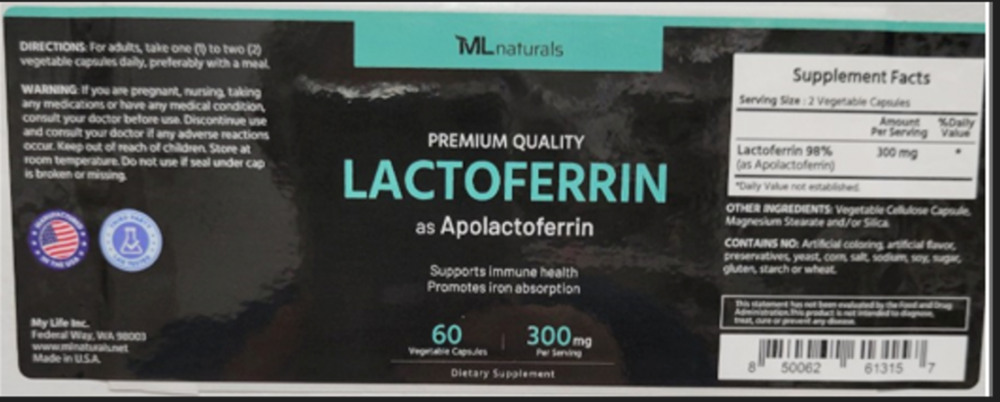
My Life Inc. of Federal Way, Washington, has pulled approximately 65 bottles of its ML Naturals Premium Quality Lactoferrin from the market after FDA inspectors discovered the product contains milk that isn’t declared on the label.
The recall specifically targets the 300mg Apolactoferrin capsules with lot number FL2407511L19 and expiration date 10/2027. The supplement comes in black plastic bottles with black lids, containing 60 vegetable capsules each.
Consumers purchased this product directly through Amazon.com and the Amazon VINE Program. Those who bought it can identify it through the ASIN #BODNLMBHGG and SKU #FC-6UFC-K026.
Health Risks for Allergy Sufferers
For people with milk allergies, this undeclared ingredient creates significant danger. Milk allergies can trigger severe, potentially life-threatening reactions in sensitive individuals.
“People who have an allergy or severe sensitivity to milk run the risk of serious or life-threatening allergic reaction if they consume this product,” the company stated in its recall notice.
Similar Posts:
Allergic reactions to milk can include hives, wheezing, digestive problems, and in severe cases, anaphylaxis—a dangerous condition that can cause throat swelling, breathing difficulties, and a dramatic drop in blood pressure.
No illnesses connected to this product have been reported so far, but the recall aims to prevent potential harm.
Company Response and Consumer Action
My Life Inc. has acknowledged the issue and committed to making changes. “ML Naturals takes the safety and trust of our customers very seriously. We are taking immediate steps to ensure all labeling and manufacturing processes prevent such issues in the future,” the company stated.
Consumers who have purchased the affected product and have milk allergies or sensitivities should not consume it. They can either return it for a full refund or dispose of it safely. Questions about returns, refunds, or replacements should be directed to [email protected].
Regulatory Context
The FDA discovered the problem during an inspection, highlighting the agency’s role in protecting consumers from undeclared allergens. This recall is being conducted with the FDA’s knowledge and oversight.
Food and supplement recalls due to undeclared allergens remain a persistent safety concern. Milk is one of the eight major allergens that must be clearly labeled under U.S. food safety regulations, along with eggs, fish, shellfish, tree nuts, peanuts, wheat, and soybeans.

Unlike prescription medications, dietary supplements don’t require FDA approval before going to market. The responsibility falls on manufacturers to ensure their products are safe and properly labeled.
This recall highlights the importance of accurate ingredient labeling, particularly for products consumed regularly like dietary supplements. For allergy sufferers, clear and accurate labels aren’t just a convenience—they’re a critical safety measure.