Carbon dioxide (CO2) can be used as a raw material for producing formic acid or methanol rather than releasing it into the atmosphere, which would exacerbate climate change. The conversion of CO2 using nanodiamonds as an environmentally friendly photocatalyst has already been researched in laboratory studies, but the process needs to be brought closer to real-world application. The nanodiamonds used are not expensive jewelry-grade diamonds, but rather detonation diamonds, which are produced on an industrial scale and are therefore relatively cheap. The diamond catalyst is “green” because it is made largely of carbon.
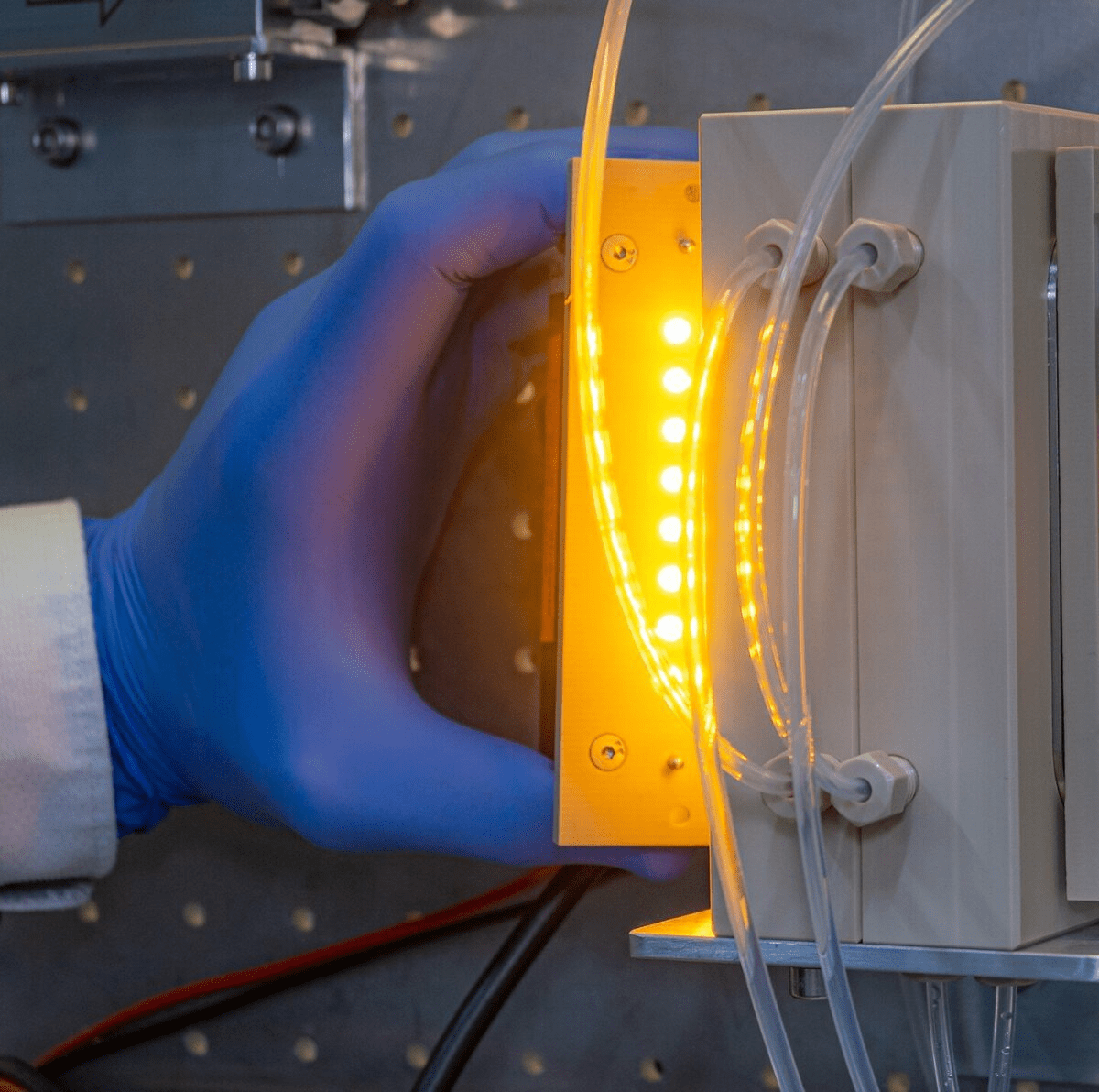
Dr. Thomas Rehm, one of the scientists at Fraunhofer IMM, says that experiments have so far been carried out in a stirred flask, which has certain disadvantages. Firstly, the catalyst, which is the nanoparticles that are floating around, needs to be separated from the solution after the reaction. Secondly, the contacting between the gas and liquid phase and the catalyst is less than ideal.
Similar Posts
The DIACAT consortium has examined various nanodiamond materials and found that even the weaker energy of blue sunlight can excite nanoparticles if there are enough hydrogen atoms on their surface. Future nanodiamond-based photocatalysts could be able to use sunlight to transform CO2 or N2 into hydrocarbons or ammonia. The potential of nanocrystalline materials as catalysts is enormous because they provide very large surfaces in relation to their volume.
The researchers have developed a microreactor with an upright standing reaction plate that features microchannels coated with the diamond catalyst. The CO2 is directed over the reaction plate from below in a counterflow configuration. By applying much higher quantities of carbon dioxide directly to the catalyst film, they can improve the gas-liquid-solid contacting, which results in a larger quantity of formic acid.
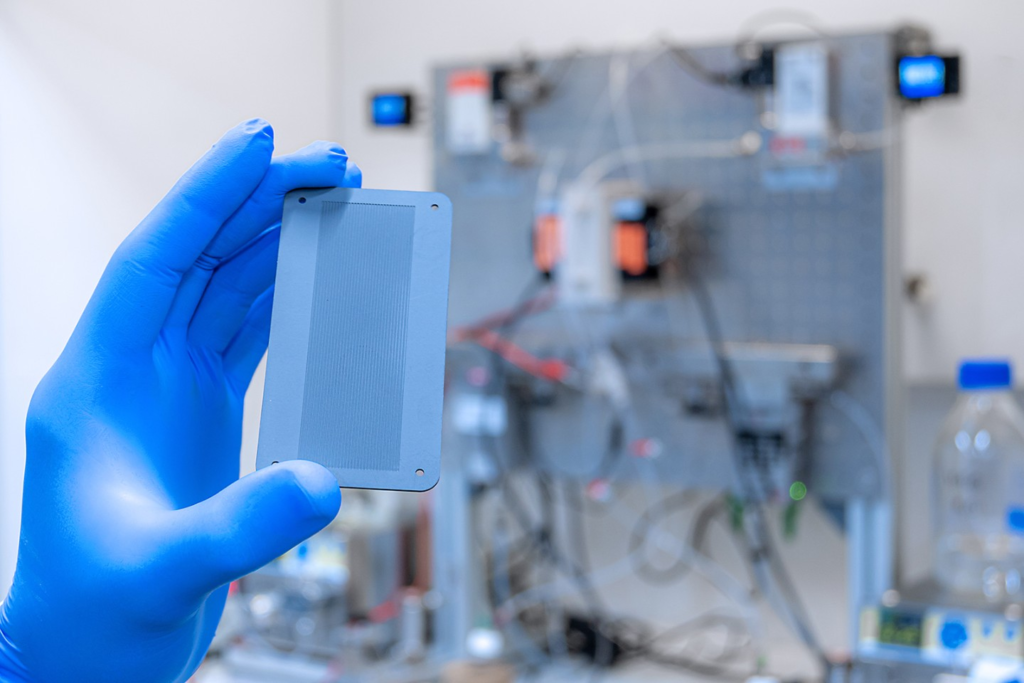
Aqueous nanodiamond dispersions with various surface terminations, such as hydrogen, -OH, or -COOH, were investigated in the Laserlab at HZB after being excited by ultrafast laser pulses. It was discovered that a certain combination of hydrogen and fullerene-like carbon on the surfaces of the nanoparticles is ideal. The hydrogen on the surfaces makes electron emission much easier.
Using CO2 as a raw material for producing formic acid or methanol is an efficient way to avoid releasing CO2 into the atmosphere and exacerbating the problem of climate change. The use of nanodiamonds as an environmentally friendly photocatalyst is relatively inexpensive and does not require costly jewelry-grade diamonds. The researchers are developing a microreactor that can apply much higher quantities of carbon dioxide directly to the catalyst film, resulting in a larger quantity of formic acid. In the future, nanodiamond-based photocatalysts could potentially use sunlight to transform CO2 or N2 into hydrocarbons or ammonia.