A dangerous discovery in a common emergency medicine has triggered an urgent nationwide recall. On January 24, 2025, Provepharm Inc. recalled its injectable blood pressure medication after finding black particles in sealed medicine vials.
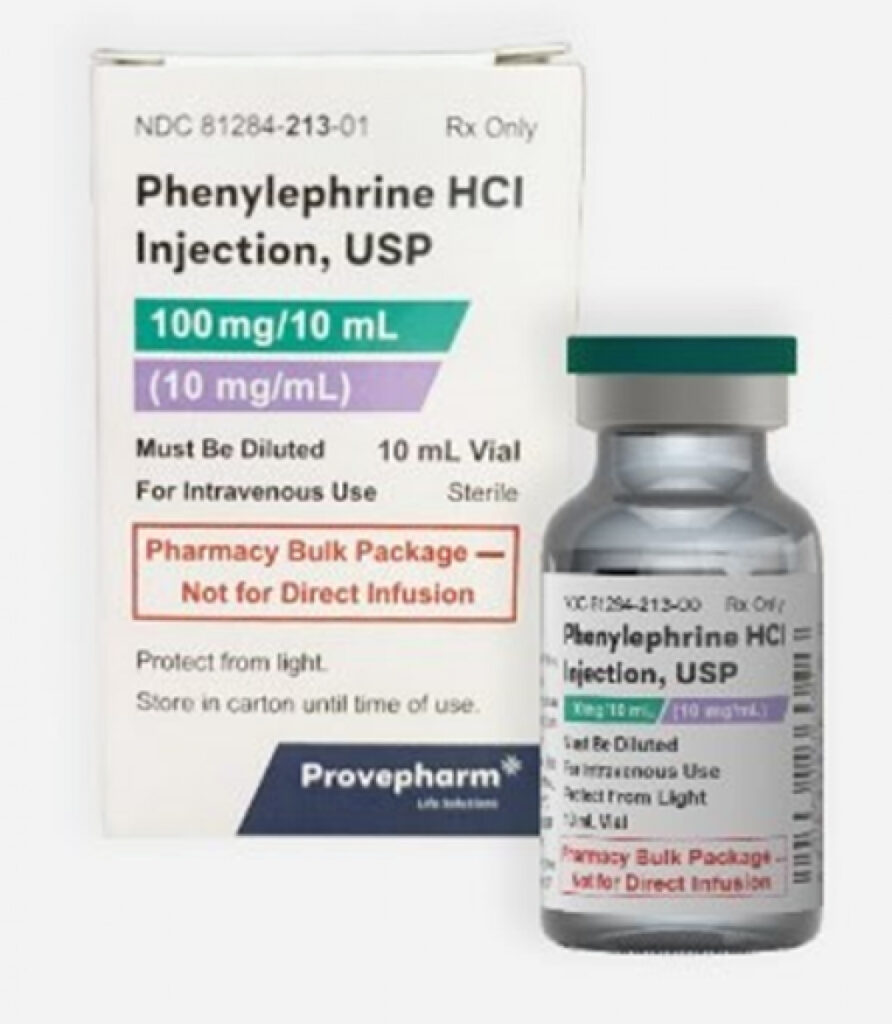
The recall affects Phenylephrine Hydrochloride Injection, a medicine doctors use during surgery to help maintain stable blood pressure. Only one batch is affected – lot number 24020027, which expires in December 2025.
According to FDA warnings, particulate matter in injectable medicines can cause serious complications. These particles can travel through blood vessels and block blood flow to important organs like the heart or brain, potentially causing serious harm including stroke or death.
Similar Posts:
This particular recall began after a pharmacy reported finding visible black particles in a single-sealed vial. The contaminated medicine was sent to hospitals and medical facilities across the country. While no one has reported getting sick from the medicine yet, wholesalers, distributors, compounding companies and hospitals must immediately cease use and return the product.
Healthcare workers can identify the recalled medicine by checking:
- The ID number (called NDC): 81284-213-01
- Lot number: 24020027
- Expiration date: December 2025
- Size: 10 mL single-dose vial
Provepharm is working with a company called Sedgwick to collect all unused vials. Hospitals should send recalled medicine to:
Sedgwick
Event## 8664
2670 Executive Drive, Suite A
Indianapolis, IN 46241
Anyone who thinks they might have had a bad reaction to this medicine should tell their doctor right away. Doctors and patients can report problems to the FDA by:
- Going online to the MedWatch program
- Calling 1-800-332-1088
- Asking for a form by mail
For questions about returning the medicine, contact Sedgwick at 866-737-5394 or email [email protected].
This recall highlights why strict quality checks are crucial when making injectable medicines. Even tiny particles can cause serious harm when injected directly into the bloodstream.